Miscellaneous bispecific antibodies
Following are some bispecific antibodies that are currently approved by the U.S. Food and Drug Administration to treat cancer. Blinatumomab (Blincyto®) is approved to treat certain types of acute lymphocytic leukemia. Amivantamab (Rybrevant®) is approved to treat some non-small cell lung cancers that have spread.
Recombinant bispecific antibodies can be divided into two classes: bispecific formats with Fc regions, and bispecific formats without Fc regions. Bispecific antibodies with an Fc region retain Fc-mediated effector functions, such as CDC and ADCC.
Bispecific antibodies have two distinct binding domains that can bind to leukemia surface proteins (including CD33 and CLL-1) on AML cells and CD3 subunits on T cells simultaneously, and trigger T-cell activation and target cell lysis.
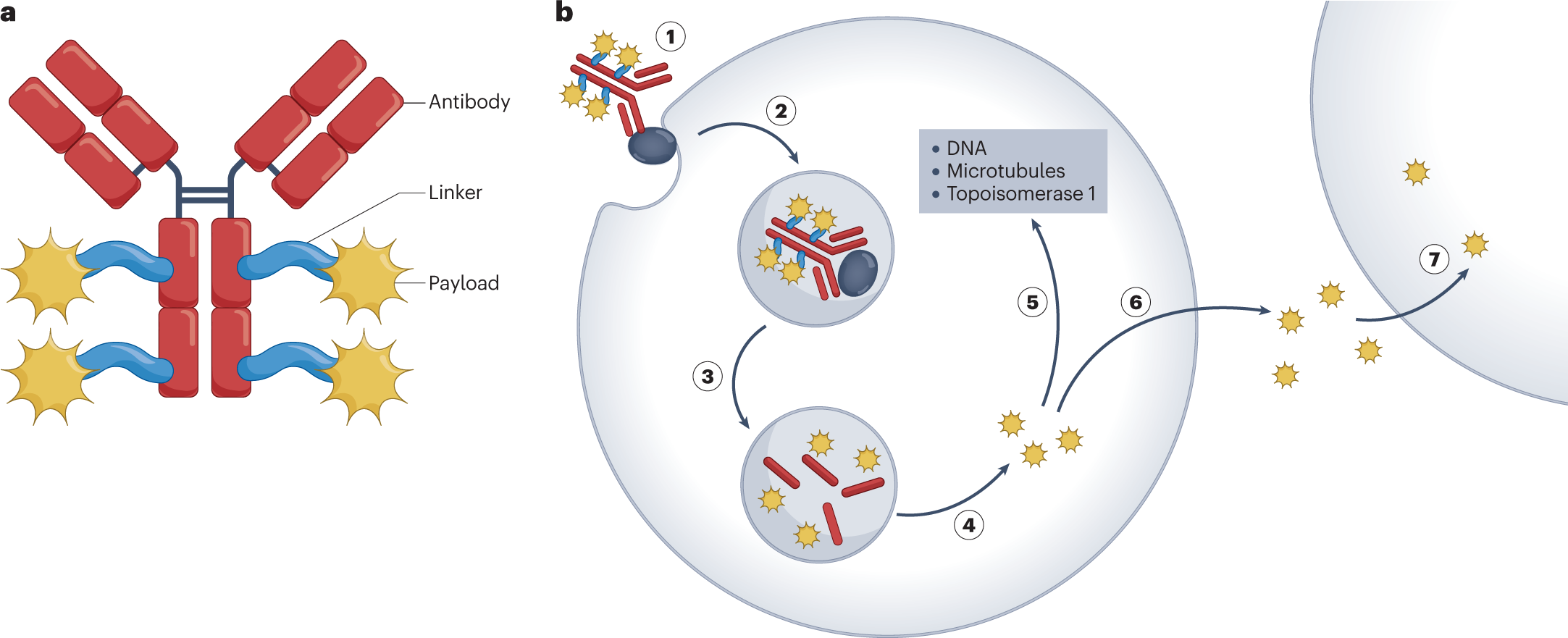
Monoclonal antibodies have two arms that each recognize the same target antigen. In contrast, bispecific antibodies are engineered hybrid molecules with two unique binding domains, each of which recognizes a unique target.
Human antibodies are classified into five isotypes (IgM, IgD, IgG, IgA, and IgE) according to their H chains, which provide each isotype with distinct characteristics and roles. IgG is the most abundant antibody isotype in the blood (plasma), accounting for 70-75% of human immunoglobulins (antibodies).

The first FDA-approved T cell-engaging bispecific antibody was blinatumomab, which was given accelerated approval for the treatment of Philadelphia (Ph) chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL) in 2014.
Bispecific antibodies (bsAbs) offer a novel approach to anticancer therapy by targeting different antigens via a range of mechanisms of action. Manipulating bsAb structures allows generation of multiple formats to optimize molecular function for specific clinical contexts.