Glycylcyclines
What are Glycylcyclines?
Glycylcyclines are a new generation of antibiotics derived from tetracyclines. They were developed to overcome issues with bacterial resistance to tetracyclines.
Glycylcycline antibiotics inhibit bacterial reproduction by blocking bacterial protein synthesis. They have broad spectrum of activity against gram-negative and gram-positive bacteria, but are more potent against bacteria that is resistance to tetracyclines. Glycylcycline antibiotics are active against resistant organisms such as methicillin resistant staphylocci, penicillin-resistant streptococcus pneumoniae and vancomycin resistant enterococci.
Glycylcyclines are broad-spectrum antibiotics used to treat bacterial infections that are resistant to tetracyclines. They are bacteriostatic (prevent reproduction in bacteria) in nature and inhibit protein synthesis by binding to the A site of the bacterial ribosomal subunit 30s.
Nausea, vomiting, and diarrhea were the most common adverse events reported with tigecycline therapy and may result in discontinuation of therapy. Resistant organisms remain a concern in hospitalized patients and are becoming an increased concern in community-acquired infections.

Presently, tigecycline is the only glycylcycline approved for antibiotic use.
Tigecycline, sold under the brand name Tygacil, is a tetracycline antibiotic medication for a number of bacterial infections. It is a glycylcycline class drug that is administered intravenously.
List of Glycylcyclines

Fig. 1. Effects of various GAR-936 (a), WAY 152,288 (b), and minocycline (c) dosage regimens on the number of S. pneumoniae ATCC 10813 organisms in the neutropenic mouse thigh muscle infection model after 48 h of treatment. The dashed horizontal line represents the number of CFU per thigh at the start of treatment. ●, single dose; ○, once-daily dose; ▾, twice-daily dose; ▿, four-times-daily dose; ——, single dose; ······, once-daily dose; –––, twice-daily dose; —··, four-times-daily dose; —–, log CFU at start of treatment.
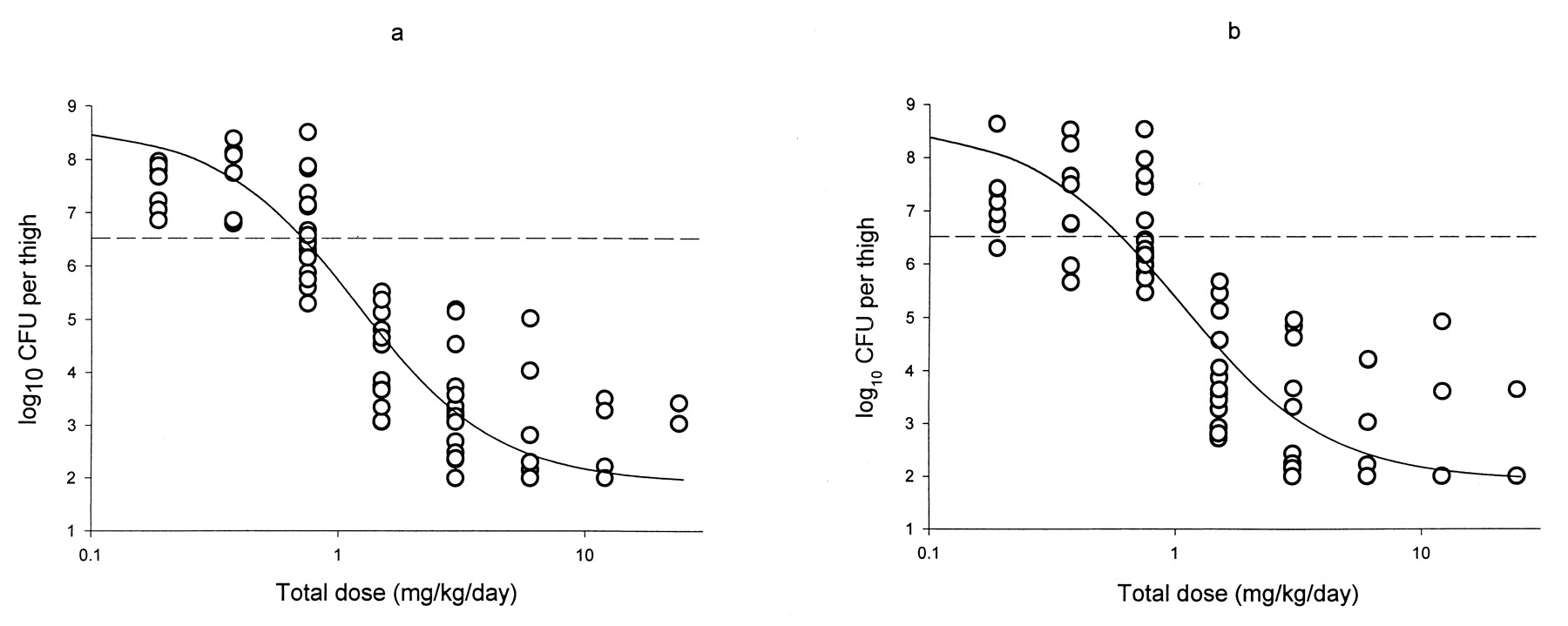
Fig. 2. Dose-effect relationship of the glycylcyclines GAR-936 (a) and WAY 152,288 (b) in an experimental thigh muscle infection withS. pneumoniae 1199 in neutropenic mice. Each point represents the results for a single mouse. The sigmoid curve represents the dose-effect curve established according to the Hill equation.
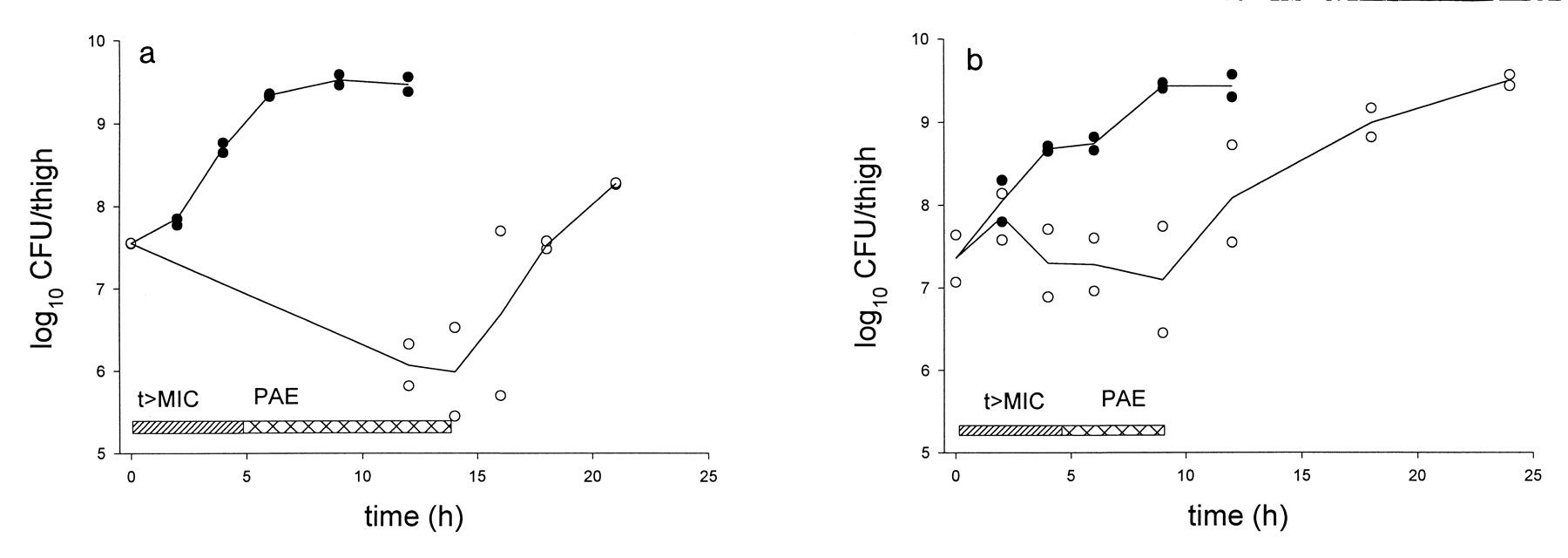
Fig. 3. In vivo PAE of GAR-936 after administration of 3 mg/kg subcutaneously to neutropenic mice infected with S. pneumoniae ATCC 10813 (a) and E. coli ATCC 25922 (b). t>MIC, period when an active concentration of GAR-936 is present; ●, untreated mice; ○, GAR-936-treated mice.

Fig. 4. Relationship between pharmacokinetic-pharmacodynamic parameters and therapeutic efficacy of GAR-936 (free drug) againstS. pneumoniae 1199 in the neutropenic mouse thigh muscle infection model (R2 = 0.82, 0.83, and 0.54 for panels a, b, and c, respectively). (a) time above the 0.5 × MIC versus effect. (b) Log AUC versus effect. (c) LogCmax versus effect.